estimation of sulphate by gravimetric method|how to calculate gravimetric sulfate : department Store In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will . WEBA través de Mi Tigo podrás pagar tu factura vencida o la totalidad de facturación en deuda. ¡Ten en cuenta! Las imágenes de referencia del proceso corresponden a la versión web o web móvil de la aplicación, .
{plog:ftitle_list}
Velozes & Furiosos 10 é um filme dirigido por Louis Leterrier .
From the weight of the sample and weight of the precipitate, the percentage of sulfate in the sample is calculated. The precipitation reaction is the following: \ [Ba^ {2+} (aq) + SO_4^ {2-} (aq) \rightarrow BaSO_4 (s)\]In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will . In this experiment, the percentage by mass of sulfate in an unknown sulfate salt will be determined by gravimetric analysis. First, a pre-weighed sample of the unknown . This document describes a procedure for determining the amount of sulfate in an unknown sample using gravimetric analysis with barium sulfate precipitation. Key steps include: 1) Adding barium chloride to the sample to .
One example of the gravimetric methods is to measure the weight of a porcelain dish where as much as 0,50 g of a sample that has been heated in an oven at temperature of 110-120 for 2 hours, is.
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown .Gravimetric Determination of Soluble Sulfate. The quantitative determination of sulfate ions in inorganic compounds can be accomplished by using the selective precipitation of the sulfate .Gravimetric Method with Ignition of Residue. Principle. Sulphate is precipitated in hydrochloric acid medium as barium sulphates by the addition of barium chloride.
The gravimetric method of sulphate ions determination is based on the precipitation reaction with BaCl2: SO4 2- + BaCl2 BaSO4 + 2 Cl- (1) when a white precipitate of BaSO4 is formed, which .
Gravimetric analysis is a quantitative determination of the amount of analyte through a precipitation process, precipitate isolation, and determination of isolated product weight. Gravimetric Determination of a Sulfate. In this experiment, we will learn the techniques associated with gravimetric analysis, and use stoichiometry to calculate the . 3. Gravimetric method with drying of residue If organic matter is not present in the sample first method can be done without igniting and instead drying the residue and weighing. Turbidimetric method Turbidimetric method .What is Gravimetric Analysis? Gravimetric analysis is a method in analytical chemistry to determine the quantity of an analyte based on the mass of a solid. Example: Measuring the solids suspended in the water sample – Once a .
In this video we had discussed about Estimation of Barium Sulphate by Gravimetric Analysis1. Principle of Estimation of Barium Sulphate by Gravimetric Analys.4) Suppose that a small portion of the sulfate precipitated as lead sulfate rather than as barium sulfate. How this would change the result of the analysis? 5) From the following list, identify the interfering species in the sulfate determination method used in this experiment: Pb2+, Na+, NO 3-, CO 3 2-, PO 4 3-.Gravimetric analysis describes a set of methods used in analytical chemistry for the quantitative determination of an analyte (the ion being analyzed) based on its mass. The principle of this type of analysis is that once an ion's mass has been determined as a unique compound, that known measurement can then be used to determine the same analyte's mass in a mixture, as long as .The method should be more widely adopted in routine clinical work. SUMMARY. The photo-electric absorptiometer and copper sulphate methods were used to determine the haemoglobin concentrations of 131 specimens of blood. The results obtained showed a strong correlation. The copper sulphate method was found to be simple and is commended for use in
Ask the Chatbot a Question Ask the Chatbot a Question gravimetric analysis, a method of quantitative chemical analysis in which the constituent sought is converted into a substance (of known composition) that can be separated from the sample and weighed. The steps commonly followed in gravimetric analysis are (1) preparation of a solution containing a known weight of .
sulfate determination chemistry
how to weigh sulfate by difference
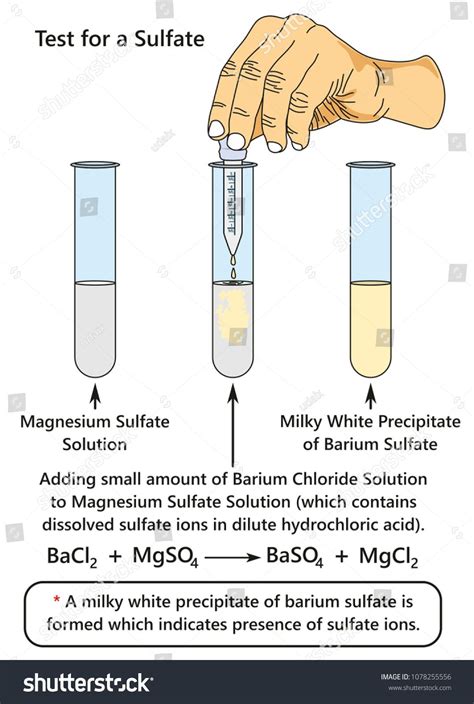

2. Suppose that a small portion of the sulfate precipitated as sodium sulfate rather than as barium sulfate. How would this affect the result of the analysis? 3. From the following list, identify the interfering species for the sulfate determination method used in this experiment: Mg2+, Pb2+, Na+, NO3-, Cl-, PO43-.
The results are compared with the weight of natural soil to determine the percent of sulfates in solution. U.S. Army and Air Force Method Gravimetric Method The U.S. Army and Air Force method-TM 5-822-14/AFJMAN 32-1019 (47) uses a gravimetric technique to determine the concentration of sulfates in solution.
The Gravimetric Estimation of Barium: The given barium chloride solution is made up to a definite volume. A measured volume of it is then treated with dilute sulphuric acid and then treated with dilute sulphuric acid and barium precipitated as barium sulphate. The precipitated barium sulphate is separated and weighed.
For example, one standard gravimetric method for the determination of magnesium involves its precipitation as MgNH 4 PO 4 •6H 2 O. Unfortunately, this precipitate is difficult to dry at lower temperatures without losing an inconsistent amount of hydrated water and ammonia. . Other standard methods for the determination of sulfate in water .
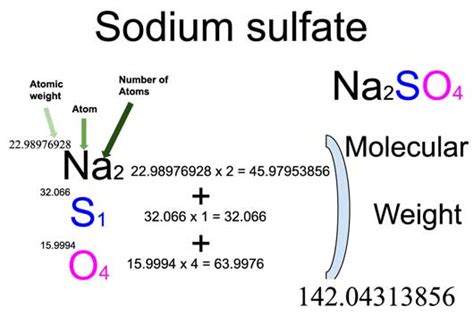
METHOD 9038 SULFATE (TURBIDIMETRIC) 1.0 SCOPE AND APPLICATION 1.1 This method is applicable to ground water, drinking and surface waters, and domestic and industrial wastes. 1.2 This method is suitable for all concentration ranges of sulfate (SO 4-2); however, in order to obtain reliable readings, use a sample aliquota) Chemical analysis by gravimetric and volumetric method, and b) Atomic absorption method. The gravimetric and volumetric methods are suitable for determination of silica, barium oxide, manganese, iron, phosphorus, sulphur, alumina and other elements in manganese ores and concentrates. The atomic absorption method can be used for the Gravimetric analysis is an analytical technique used for the quantitative determination of an analyte based on the mass of a solid. . to determine the sulphate ions (SO 4 2-) contained in ammonium sulphate (NH 4) . This method has been successfully used to estimate a wider range of organic compounds, including lactose in milk products .Reduced volume versions of this method that use the same reagents and molar ratios are acceptable provided they meet the quality control and performance requirements stated in the method. 2.3. Limited performance-based method modifications may be acceptable provided they are fully documented and meet or exceed requirements expressed in
• Purity of the precipitate: co-precipitation and post precipitation, • Estimation of barium sulphate 11/1/2018 Deokate U A 2 3. . • Gravimetric method is one in which the analysis is completed by a weighing operation. • .Gravimetric methods: The . quantitative methods. that are based on determining the . mass. of a . pure compound . to which the . analyte. is . chemically related. • Precipitation gravimetry: The . analyte. is separated from a solution of the sample as a . precipitate. and is converted to a compound of known composition that can be weighed .
Nevertheless, a gravimetric method could work with any reaction producing precipitate. In the nineteenth century and earlier, many precipitations gravimetric methods were developed, often to analyze ore. A total analysis method typically provides better than 0.1% accuracy, which means that the analyte is represented by 99.99% of the precipitate.The Gravimetric Estimation of Nickel: The nickel is precipitated as nickel dimethyl glyoxime by adding alcoholic solution of dimethyl glyoxime C 4 H 6 (NOH) 2 and then adding a slight excess of aqueous ammonia solution.. When the pH is buffered in the range of 5 to 9, the formation of the red chelate occurs quantitatively in a solution.Decomposition Method" at the end of this document) Figure 1. Microwave carousel and vessels. The lid torque tool is labeled A. NOTE: Label tape must NOT be applied directly to the Teflon vessel, but should be used on the fiber vessel sleeves. 1. Accurately weigh three 1.0 to 1.2-g samples of the dried unknown into clean, dry Teflon PFA
The classic technique for sulfate analysis in an undergraduate quantitative analysis lab involves precipitation as the barium salt with barium chloride, collection of the precipitate by gravity .Estimation of haemoglobin by the copper sulphate gravimetric method. Estimation of haemoglobin by the copper sulphate gravimetric method Glasgow Med J. 1947 Jan;28(1):28-31. Author M H MILLER. PMID: 20288230 PMCID: PMC5969648 No abstract available. MeSH terms Copper Sulfate* .The purpose of the experiment is to quantitatively determine the amount of sulphate, in barium sulphate, by the gravimetric method. (C) Theory. Gravimetric analysis gets its name from the process of isolating the desired constituent in weighable form. In summary, it involves changing one compound containing the constituent into another compound .
Background: The National Blood Transfusion Service (NBTS) in Tanzania uses the Copper Sulphate (CuSO4) gravimetric method to estimate hemoglobin (Hb) in blood donors. However, this and other point-of-care methods, including HemoCue, may provide false results. Therefore, this study aimed to evaluate the performance of CuSO4 and HemoCue methods .òSulphate Standard for Turbidimetric / Gravimetric Method ó. (This solution will be available in the laboratory). ¾ Add 50 mL water to 50 mL concentrated HCl. Label, ò1+1 HCl solution ó. . 8B Estimation of Sulphate in Water A. Lab Report: (4 x 10 Marks = 40 Marks) Data: A Mass of unknown sample containing sulphate: 0.6039 g
The gravimetric method of sulphate ions determination is based on the precipitation reaction with BaCl 2: SO 4 2-+ BaCl 2 . Although, the gravimetric method is mentioned in Romanian standard for sulphate analysis (STAS 8601-70), its use is not always handy because it requires a long working time and relatively high concentrations of sulphate .
The method should be more widely adopted in routine clinical work. SUMMARY. The photo-electric absorptiometer and copper sulphate methods were used to determine the haemoglobin concentrations of 131 specimens of blood. The results obtained showed a strong correlation. The copper sulphate method was found to be simple and is commended for use in
how to calculate sulfate weight
how to calculate gravimetric sulfate
TNAFLIX free porn videos. Vicky Soleil's Breast Exam - Dee Dee (Jay Dee, Moxxy Minx, Lizzy Xxx, Dawn Knudsen, Dorian Dennis, Nipple Play)
estimation of sulphate by gravimetric method|how to calculate gravimetric sulfate